Periodic Table of the Elements: Difference between revisions
| Line 89: | Line 89: | ||
<center>'''''But where did water ( H<sub>2</sub>O ), olivine ( ( Mg, Fe )<sub>2</sub>SiO<sub>4</sub> ), and amino acids (such as C</sub>3</sub>H<sub>5</sub>NO ) come from?'''''<.center> | |||
But where did water ( | |||
When dying stars explode and make beautiful planetary nebulae and supernova remnants, they spew out heavy elements that had been fused in their cores. | When dying stars explode and make beautiful planetary nebulae and supernova remnants, they spew out heavy elements that had been fused in their cores. | ||
Revision as of 09:51, 20 February 2012
Elements
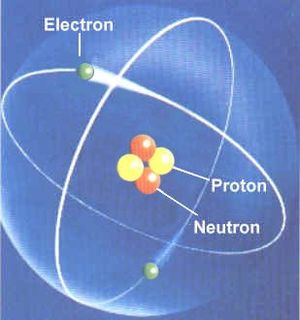
The chemical elements are the basic atoms, from which all chemical compounds are made. All chemical elements are listed in the Periodic Table of Elements. Each has an atomic number, the number of protons in that atom. Hydrogen (H) has one proton in its nucleus, Helium (He) has 2, and so on.
The atomic mass is roughly the number of protons + neutrons (plus a little more for the electrons and the fact that a neutron is slightly more massive than a proton). Most light atoms have the same number of neutrons as protons. So the atomic mass of He is (2 protons + 2 neutrons) roughly 4 (plus a little more). The exceptions are (1) Hydrogen, which is found most often as a proton with an electron and no neutron, and (2) the heaviest elements, which need more neutrons to glue the atom together keeping the protons from repelling each other. For example, copper has an atomic number of 29 (29 protons) but an atomic mass, not of 58 but of 63 meaning it has 34 neutrons. (Specifically one atomic mass unit equals 1/12th the mass of a carbon atom, but it is easiest just to look up the atomic mass on the Periodic Table.)
Atoms are normally neutral, that is there are the same number of protons (positive charges) in an atom, as there are electrons (negative charges). If an atom has too many or too few electrons, it is called an ion. If there are too many electrons it is a negative ion (since electrons have a negative charge.) If it has too few electrons it is a positive ion. Dissolve table salt in water and the NaCl molecule becomes Na+and Cl- in solution. In the Earth's atmosphere, N2 (nitrogen molecule) and O2 (oxygen molecule) may lose an electron when struck by a cosmic ray and become the ions N2+ and O2+. Sunlight and starlight can ionize elements and compounds. In interstellar gas, neutral H atoms may become H+, a bare proton with no electron.
If positive ions and negative ions are adjacent, and the environment is right, they will naturally combine. Another way elements can combine to form compounds is by covalent bonding, sharing electrons.
Chemical elements combine to make chemical compounds, such as water (H2O) or olivine ( ( Mg Fe )2 SiO4) and organic compounds such as amino acids which make up proteins, and nucleotide bases (purines and pyrimidines) which make up DNA and RNA ... the building blocks of Life.
An atom of helium, a chemical element
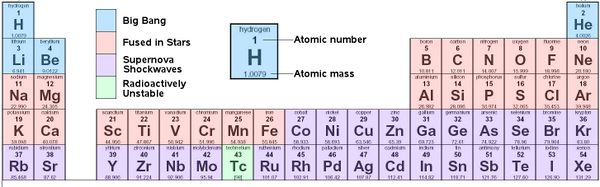
Periodic Table of Chemical Elements
Elements formed during the Big Bang, by fusion in stars, and by shockwaves in supernovae
Origins of Elements
The Big Bang
At the time of the Big Bang, pressure and temperatures were such that subatomic particles could form. As conditions changed so did the types of particles that formed. Electrons, quarks, protons and neutrons, all happened in a fraction of a second. Helium ions, 2 protons and 2 neutrons in one nucleus, were present after 3 minutes. Not until 300,000 years later was the universe cool enough to allow protons, helium and other nuclei to capture electrons to create atoms of hydrogen, and helium, and traces of lithium and beryllium.
To this day the most abundant element in the universe is still hydrogen. It shows up as a magenta color in many star forming nebulae, such as the Horsehead Nebula where hydrogen is reacting with starlight. The dark absorbing cloud that forms the familiar shape and blocks our view of stars behind the nebula, is dust, mostly made of carbon.
Exaggerated magenta-colored ionized hydrogen in the Horsehead Nebula
T.A.Rector (NOAO/AURA/NSF) and Hubble Heritage Team (STScI/AURA/NASA)
Fusion in Stars
As gravity pulled the hydrogen and helium together the earliest stars formed. Fusion inside stars would convert hydrogen (H) to more helium (He), then to carbon (C), and depending on the size of the star, the process might continue up to iron (Fe), but nothing heavier could fuse in a star and still release energy.
Stars in M 103 fusing elements
When small stars die they spew out these new heavier elements. These are caught up in later star-forming nebulae, so that solid planets may form around second and third generation stars. The carbon in the Horsehead Nebula came from inside stars.
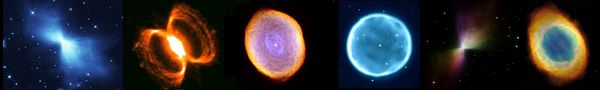
Planetary nebulae from dying stars throwing off their outer layers
Shock Waves in Supernovae
When giant stars die, they not only send out elements up to iron, but in the shockwaves from their explosive supernova, create elements heavier than iron, such as silver (Ag), gold (Au), and platinum (Pt).
Supernova remnants: Crab Nebula and remnants from a pair of Supernovae DEM L316
Credit: Gemini Observatory
In the dense early Universe the first stars would have been huge. The larger the stars, the more gravitational pressure, and the faster they use up their fuel. These giant stars lasted only a few million years, then exploded in violent supernovae. Supernova remnants would fly out into space, to be caught up by later generations of stars. Copper (Cu), zinc (Zn), and iodine (I), were among the elements made in supernovae, elements which in trace amounts are necessary for Life.
Vela supernova remnants
Every living thing is made of bits of Big Bang (H), chemicals fused in stars (O) (C), and bits of Supernova remnants (Cu) ! Every living thing has stardust in it.
Origins of Chemical Compounds
When dying stars explode and make beautiful planetary nebulae and supernova remnants, they spew out heavy elements that had been fused in their cores.
Dumbbell Nebula (M27), a "planetary" nebula from a dying star
Detail of the Dumbbell Nebula showing knots of heavy elements flying out from the dying star
In the intense heat inside a star, chemical compounds cannot form, but as dense clouds of gas and dust are kicked out into space they cool. When conditions and temperature are right, elements bond and create compounds. The denser the environment, the more likely elements are to collide and form compounds. This may happen either in gaseous knots, or in Bok globules in giant star-forming nebulae where dust may be seen gathering under the influence of its own gravity (below), or in protoplanetary disks where dust is collecting to form stars and their planets. We can tell what compounds are present in the molecular clouds of star forming nebulae, because patterns show up in their spectra just they do for the atomic elements.
Nebula in Large Magellanic Cloud where stars are forming
Bok Globules before they become protoplanetary disks
Protoplanetary disks, where future planets are forming around future stars, in the Orion Nebula
Water
Water (H2O)
Everyone's favorite compound is water. H2O forms naturally in space, through chemical reactions on the surface of dust grains and in cold dense gas. Water on the cold dust remains ice as long as it is cold, eventually finding its way into the disk from which planets form. NASA has an airborn observatory to study how this happens.
The processes that determine water formation and destruction depend on temperature. You know that hydrogen "burns", and in the presence of oxygen it will self ignite at 550 C (1022 F) and form water. In the protoplanetary disk, from which the solar system formed, water was present in large quantities. It was ice in the cold outer regions, and gas was where it was warmer, closer to the early Sun. Above 3000 C water will dissociate into OH and H, so there is no water in the atmosphere of the Sun or close to it. However, even when it is cold, unprotected by a planetary atmosphere or by dust in the disk, water is dissociated by sunlight. Its oxygen and hydrogen atoms are ionized, and blown out by the solar wind. Small bodies like Mercury or the Moon had too little gravity to hold on to water, and no atmosphere to prevent its dissociation and loss. In the outer solar system, water and ice were retained by some of the satellites of Jupiter and Saturn. The Oort Cloud, a remant of the solar nebula, is where icy comets still reside.
In thick protective atmospheres, water could have been held by early planets until they were cool enough to have rain. This, and comets, would continue to bring water to the busy early solar system, enough water to fill the oceans. The Earth still has its water and is warm enough for liquid oceans. Colder Mars also has its water, hidden as ice under the soil.
Olivine and Pallasite Meteorites
A pallasite meteorite
Olivine ((Mg,Fe)2SiO4) would also form in the protoplanetary nebula. If a planet or asteroid is large enough, it forms layers differentiating different minerals, with heaviest elements, usually iron, in the center. In a powerful enough collision, the iron, with a lower melting point than olivine, melts around the fragments of olivine. Together they fly out making beautiful pallasites. It is possible to get an idea of the energy in an impact of this sort by the heat that is generated, which would be above the melting point of iron, 1535 C, but not so hot as to melt olivine,1760 C.
Molten iron envelops pieces of olivine (which in its pure form is the gemstone peridot) in a pallasite meteorite
Organic Compunds such as Amino Acids and Nucleotide Bases
The Murchison Meteorite
Carbonaceous chondrites are a type of meteorite known to contain high concentrations of water and organic compounds. The Murchison Meteorite, which fell near Murchison, Australia, in 1969, has been found to contain over 100 amino acids, some rarely seen on Earth, and also purines and pyrimidines, nucleotide bases that are building blocks of DNA and RNA. This discovery shows that amino acids and components of the genetic code, were already present in the early solar system, available not only to Earth but to other planets and moons as well.
In addition to organic chemicals coming via meteorites, seventeen of the twenty amino acids have been produced in the laboratory, in experiments simulating the early Earth, complete with water, heat, and an electric spark for lightning.
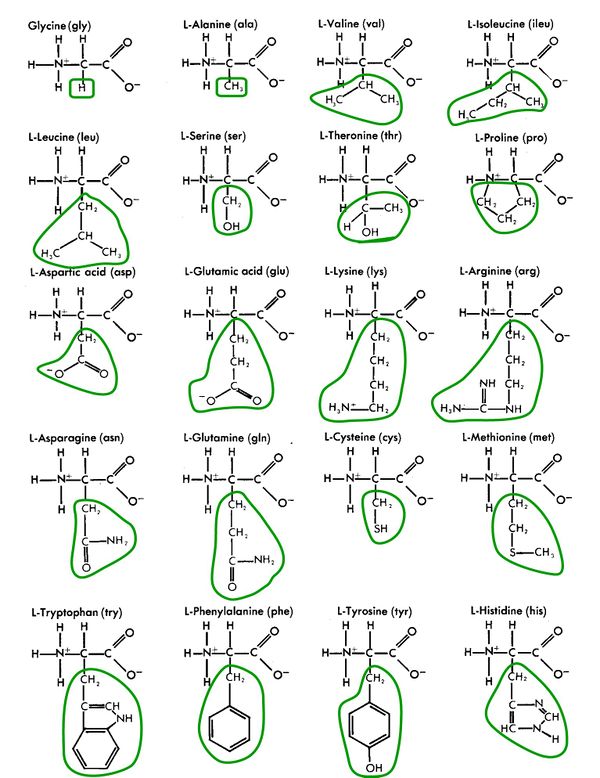
Amino acids are made up simply of hydrogen (H), carbon (C), oxygen (O), and nitrogen (N), and occasionally sulfur (S).
Amino acids are alike and different
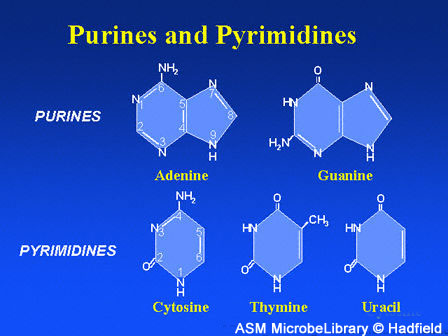
While amino acids are the building blocks of proteins, purines and pyrimidines (also called nucleobases or nucleotide bases) are the building blocks of DNA and RNA. They, also, are simple compounds of hydrogen (H), carbon (C), oxygen (O), and nitrogen (N), all found in the interstellar gas from which stars and planets form.
Purines and pyrimidines are building blocks of DNA and RNA
These nucleotide bases are abbreviated A,G,C, T, and U.
A, G, C, T make DNA
A, G, C, U make RNA
When strung together they make the genetic code: all of which formed in the early solar nebula, and all of which could have landed on any planet or moon. Since these happened around our star, it is likely that organic molecules formed around other stars as well, making life a definite possibility elsewhere in the Universe.